Dec 29, 2023We substitute this value of [O] into the rate expression [O][O 3] for Step 2, which yields the experimentally-obtained rate law. rate = k1K[O3]2 [O2] Example 17.4.4: A reaction having an apparently Negative Ea. Consider the gas-phase oxidation of nitric oxide: 2NO + O 2 → 2NO 2.
a. Draw the mechanism for the following reaction if it involves s… | Channels for Pearson+
The catalyst can be identified by the fact that it is not used up or consumed in the reaction. So, we want to look for something that is present as a reactant, and also as a product, i.e. is not consumed or changed. In the present example, we have Mn 2+ as a reactant in Step 1 but it appears as a product in Step 3 so it has not been consumed or

Source Image: chegg.com
Download Image
Key points A catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process. Catalysts typically speed up a reaction by reducing the activation energy or changing the reaction mechanism. Enzymes are proteins that act as catalysts in biochemical reactions.

Source Image: pinterest.com
Download Image
Bromination [Br2 plus catalyst] – ChemistryScore A catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower activation energy than for the uncatalyzed reaction.

Source Image: youtube.com
Download Image
For The Following Reaction Mechanism What Is The Catalyst
A catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower activation energy than for the uncatalyzed reaction. A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. Such catalysts generally function by furnishing an active surface upon which a reaction can occur. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas
Practice Identifying Catalysts and Intermediates – YouTube
A catalyst works by providing a different pathway for the reaction, one that has a lower activation energy than the uncatalyzed pathway. This lower activation energy means that a larger fraction of collisions are successful at a given temperature, leading to an increased reaction rate. Created by Jay. Reaction mechanisms using a bifunctional catalyst (metal-acid/sites) [12]. | Download Scientific Diagram
![Reaction mechanisms using a bifunctional catalyst (metal-acid/sites) [12]. | Download Scientific Diagram](https://www.researchgate.net/publication/290439645/figure/fig2/AS:318092136730625@1452850483460/Reaction-mechanisms-using-a-bifunctional-catalyst-metal-acid-sites-12.png)
Source Image: researchgate.net
Download Image
Perkin Reaction (Perkin Condensation): definition| mechanism| example| application « Organic Chemistry Reaction A catalyst works by providing a different pathway for the reaction, one that has a lower activation energy than the uncatalyzed pathway. This lower activation energy means that a larger fraction of collisions are successful at a given temperature, leading to an increased reaction rate. Created by Jay.
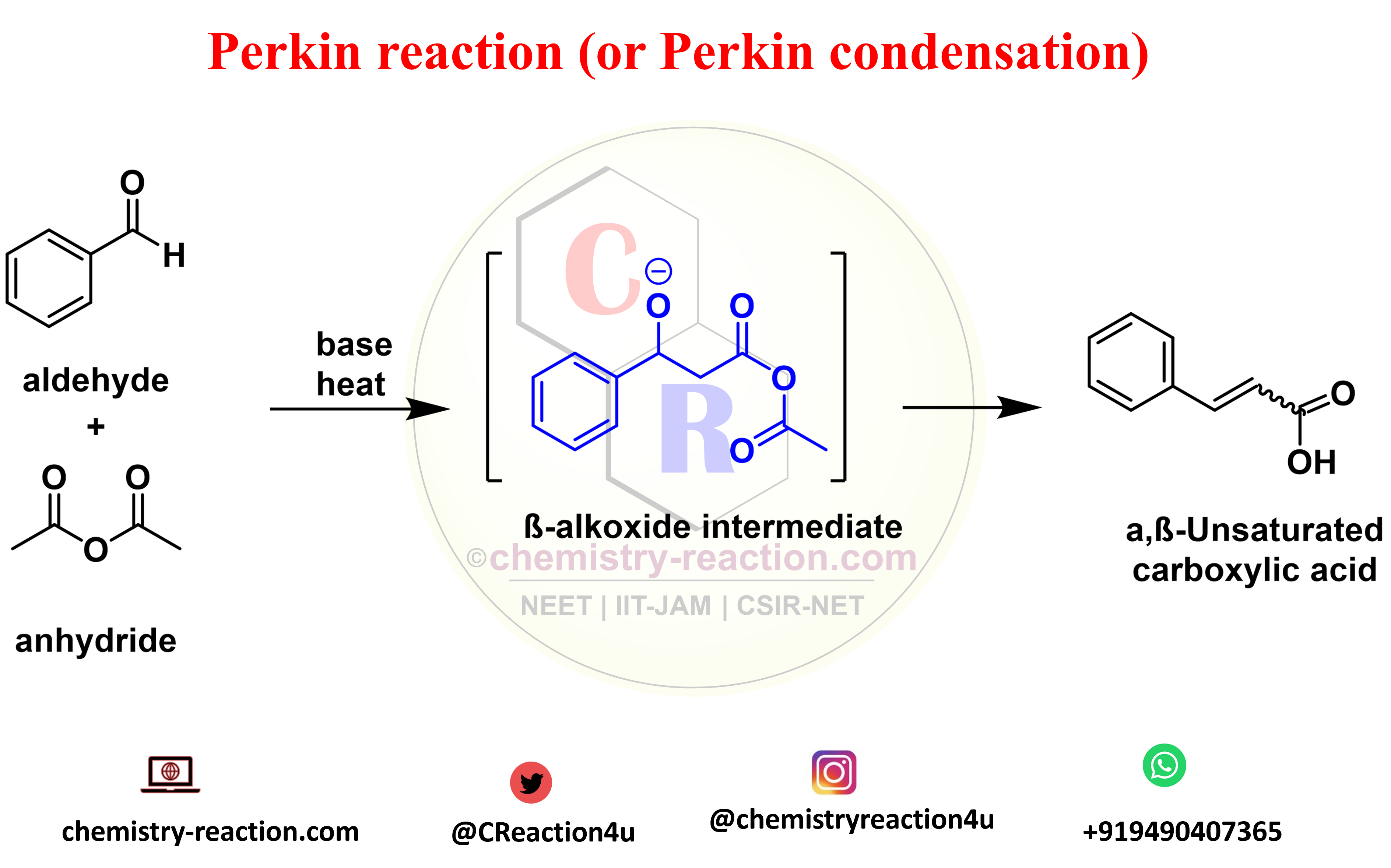
Source Image: chemistry-reaction.com
Download Image
a. Draw the mechanism for the following reaction if it involves s… | Channels for Pearson+ Dec 29, 2023We substitute this value of [O] into the rate expression [O][O 3] for Step 2, which yields the experimentally-obtained rate law. rate = k1K[O3]2 [O2] Example 17.4.4: A reaction having an apparently Negative Ea. Consider the gas-phase oxidation of nitric oxide: 2NO + O 2 → 2NO 2.

Source Image: pearson.com
Download Image
Bromination [Br2 plus catalyst] – ChemistryScore Key points A catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process. Catalysts typically speed up a reaction by reducing the activation energy or changing the reaction mechanism. Enzymes are proteins that act as catalysts in biochemical reactions.
![Bromination [Br2 plus catalyst] - ChemistryScore](https://chemistryscore.com/wp-content/uploads/2019/11/Bromination1-768x379.png)
Source Image: chemistryscore.com
Download Image
Catalytic Reaction Mechanism – an overview | ScienceDirect Topics The reaction mechanisms, however, are clearly different. The uncatalyzed reaction proceeds via a one-step mechanism (one transition state observed), whereas the catalyzed reaction follows a two-step mechanism (two transition states observed) with a notably lesser activation energy. This difference illustrates the means by which a catalyst

Source Image: sciencedirect.com
Download Image
New insights into the mechanism of Schiff base synthesis from aromatic amines in the absence of acid catalyst or polar solvents [PeerJ] A catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower activation energy than for the uncatalyzed reaction.
![New insights into the mechanism of Schiff base synthesis from aromatic amines in the absence of acid catalyst or polar solvents [PeerJ]](https://dfzljdn9uc3pi.cloudfront.net/2020/ochem-4/1/fig-3-full.png)
Source Image: peerj.com
Download Image
3.6: Day 23- Reaction Mechanisms – Chemistry LibreTexts A heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. Such catalysts generally function by furnishing an active surface upon which a reaction can occur. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas
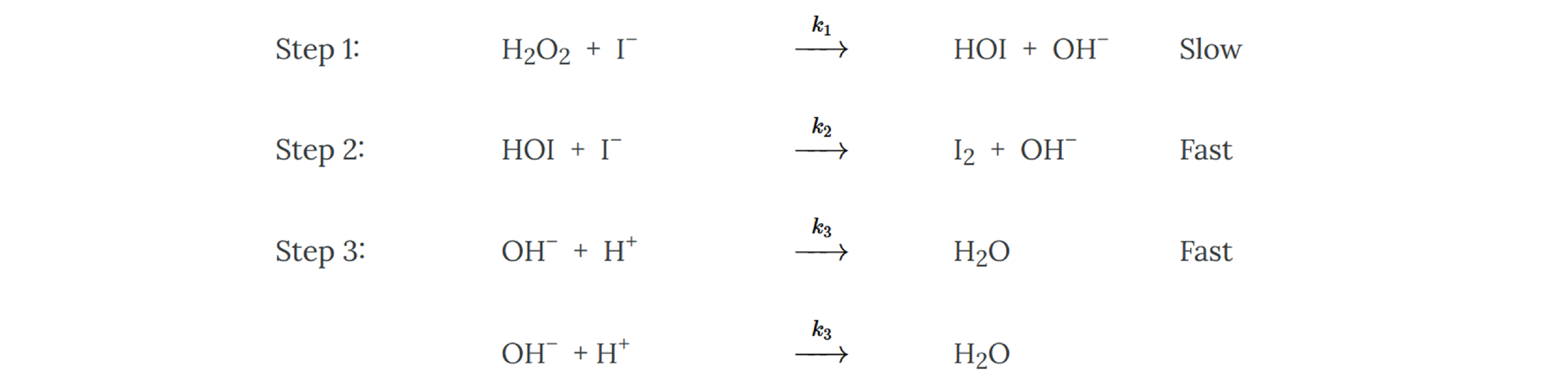
Source Image: chem.libretexts.org
Download Image
Perkin Reaction (Perkin Condensation): definition| mechanism| example| application « Organic Chemistry Reaction
3.6: Day 23- Reaction Mechanisms – Chemistry LibreTexts The catalyst can be identified by the fact that it is not used up or consumed in the reaction. So, we want to look for something that is present as a reactant, and also as a product, i.e. is not consumed or changed. In the present example, we have Mn 2+ as a reactant in Step 1 but it appears as a product in Step 3 so it has not been consumed or
Bromination [Br2 plus catalyst] – ChemistryScore New insights into the mechanism of Schiff base synthesis from aromatic amines in the absence of acid catalyst or polar solvents [PeerJ] The reaction mechanisms, however, are clearly different. The uncatalyzed reaction proceeds via a one-step mechanism (one transition state observed), whereas the catalyzed reaction follows a two-step mechanism (two transition states observed) with a notably lesser activation energy. This difference illustrates the means by which a catalyst